
- This event has passed.
Webinar – Computer Simulations (FEA, CFD) for Life Sciences and Medical devices
2 May, 2024 @ 10:00 am - 11:00 am
For European companies or institutes –
4RealSim welcomes you to attend our webinar How to benefit from computer simulations for life sciences and medical devices.
Computer modelling and simulation
Computer Modeling and Simulating (CM&S) has the potential to accelerate the long and challenging development process of medical devices and to move faster to the market, while reducing significantly costs associated with development and V&V activities. International regulatory agencies like EMA or FDA are recognizing the vast opportunities of making CM&S the fourth column in the medical device development process, besides bench-top testing, in-vitro, and in-vivo studies.
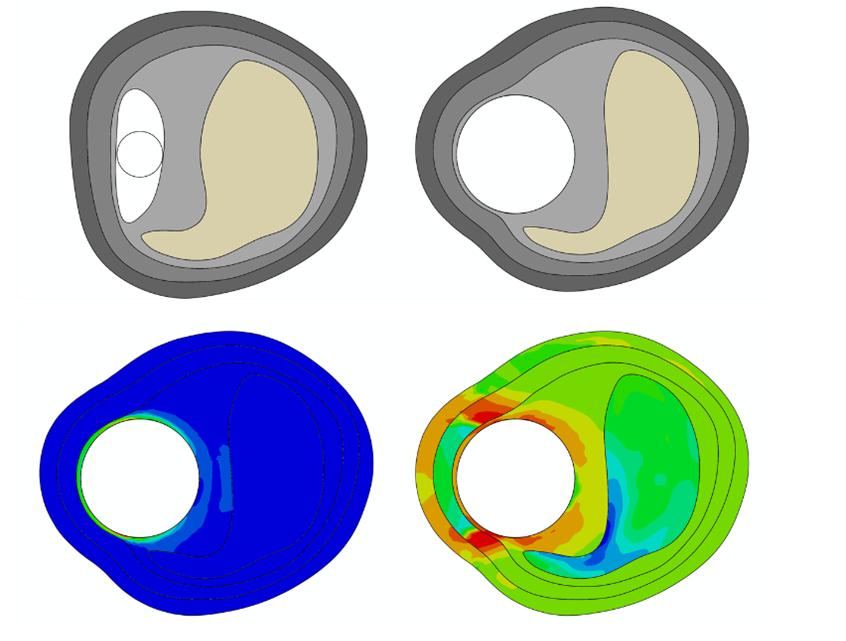
4RealSim Life Sciences Computer modeling solutions
4RealSim Life Sciences is a leading service provider in that field and offers a large array of sophisticated multi-physics simulation processes & tools that support all phases of the medical device development process – from virtual feasibility studies via classic device verification and validation analyses to augmenting your human clinical studies with corresponding modelling and simulations – the in-silico trial. We offer in the simulation services for the following physical domains:
- Structural Mechanical Analyses
- Computational Fluid Dynamics
- Fluid-Structure Interaction
- Heat-Transfer Analyses
- Coupled Heat-Stress Analyses
- Electromagnetic Analyses
4RealSim Life Sciences has experience in conducting engineering services in the field of
- Stents
- Tissue modelling
- Surgical Equipment
- Orthopedic implants (knee, hip, …)
- Medical devices
Intended for
- Medical device manufacturers
- Quality engineers
Topics
- Computer Modelling and Simulation in the Medical Device Industry
- How to support the product development process?
- Verification & Validation of Computer Modelling in Medical Device Industry
- Benefits ASTM/ISO compliance
- Industrial applications
Who is 4RealSim?
4RealSim Life Sciences is a leading service provider in that field and offers a large array of sophisticated multi-physics simulation processes & tools that support all phases of the medical device development process – from virtual feasibility studies via classic device verification and validation analyses to augmenting your human clinical studies with corresponding modelling and simulations – the in-silico trial.
4RealSim is part of the EU-funded SimInSitu project.
4RealSim is part of The Living Heart Project.


